Am I a data controller? GDPR for social media users explained

We look at the UK GDPR and the Data Protection Act 2018 and outline how the GDPR can apply to both organisations and individuals as data controllers.
Read more
We make the difference. Talk to us: 0333 004 4488 | hello@brabners.com
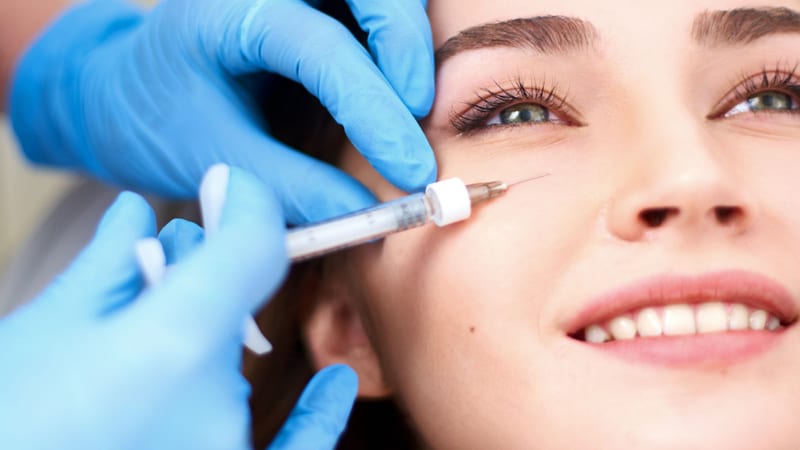
Non-surgical cosmetic procedures are gaining significant popularity, with dermal fillers being one of the most sought-after treatments.
While the obvious benefit of a non-surgical procedure is that patients avoid going ‘under the knife’, the surge in body image dissatisfaction has created a vacant space for market growth, which currently lacks substantial regulation.
Here, we explore the state of patient safety in the cosmetics industry and outlines the various regulatory measures being considered by the UK Government.
In February 2022, the Department of Health and Social Care Committee (HSCC) issued a report on the impact of body image and mental and physical health. This recommended the introduction of a regulatory regime for non-surgical cosmetic procedures.
Notably, the findings revealed that:
Furthermore, in its evidence to the HSCC, the Joint Council for Cosmetic Practitioners (JCCP) — the self-regulator for the non-surgical aesthetics industry — reported that, of those who sought non-surgical procedures:
More recently, the UK’s largest academic survey on the complications arising from Botulinum Toxin (Botox) was conducted. Comprising of 511 participants who underwent treatment between 1999 and 2023, the Skin Health and Disease journal reported that:
Such high figures further indicate the likelihood of new regulatory measures being introduced in the name of patient safety.
Within its response to the HSCC report, the Government stated that it is committed to patient safety measures and willing to focus efforts on safeguarding individuals from potential harm.
Some of these measures include:
The Government agrees with the HSCC's recommendation for a licensing regime for non-surgical cosmetic procedures. In April 2022, the Health and Care Act gave the Secretary of State for Health and Social Care the power to introduce a licensing regime. With details yet to be released, there is no immediate requirement for licenses until further consultations are held.
In response to the potential risks faced by vulnerable groups, the Government is considering a proposal for a two-part consent process. This would involve a full medical and mental health history and a mandatory 48-hour cooling-off period between consent and the procedure processes.
The HSCC had called for mandatory mental health screening to be included in the education and training framework for practitioners. In that regard, Professor David Sines of JCCP said “there should be no assessment without psychological and emotional assessment and safety nets must be built into the context of the pre-treatment, the treatment and the post-treatment plan for aftercare”.
Currently, there are no specific premises standards for beauty salons and non-CQC (Care Quality Commission) registered premises that provide non-surgical cosmetic procedures.
The Government acknowledges the need for premises standards but is mindful of duplicating existing inspection regimes. As such, it plans to liaise with the CQC to ensure that any “new premises standards operate consistently with regulatory frameworks already in place”.
The government agrees with the HSCC’s recommendation for a minimum standard to be met in respect of the education, training and qualification of individuals who perform non-surgical cosmetic procedures.
The JCCP has already commenced drafting a competency framework that covers high-risk non-surgical cosmetic procedures. In addition to several Ofqual-approved qualifications, the government has stated that it will continue to work with JCCP and other stakeholders to identify further requirements.
The HSCC’s recommendation that dermal fillers should become POMs (prescription-only medicines) has been reviewed.
The Government’s response is that dermal fillers are currently treated as medical devices. However, the Medicines and Healthcare Products Regulatory Agency (MHRA) intends to introduce more stringent rules for certain aesthetic and non-medical products (including dermal fillers) under the Medical Devices Regulations 2002. As such, there are no current plans to categorise dermal fillers as POMs.
It’s certainly encouraging to see that a culture of responsibility and transparency is being promoted in the beauty industry. The changes will inevitably impact all practitioners and salons involved in cosmetic work, though the finer details of the Government’s proposed plans are yet to be released.

We look at the UK GDPR and the Data Protection Act 2018 and outline how the GDPR can apply to both organisations and individuals as data controllers.
Read more

We explore how World Rugby’s newest reforms are reshaping the sport and what they mean for clubs and governing bodies ahead of the 2026 Six Nations.
Read more

We explore the regulatory risks that threaten solicitors’ clients, firms and professional standing and highlight the five most common pitfalls to avoid.
Read more