Botched Botox: A wake-up call for healthcare regulation
AuthorsThorrun GovindHannah Fawcett
5 min read
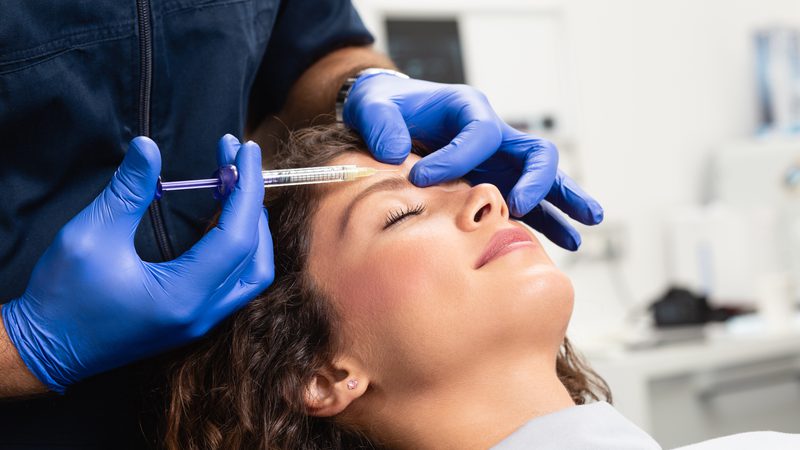
In light of the continued use of illegal beauty treatments and the introduction of new regulation around prescription-only medicines, Thorrun Govind and Hannah Fawcett from our Beauty & Fashion team call for comprehensive reform.
A series of incidents involving an aesthetic practitioner from County Durham, reported recently by the BBC, has reignited concerns about the regulation of non-surgical cosmetic treatments in the UK. The practitioner administered an unlicensed botulinum toxin product known as Toxpia to multiple clients, resulting in at least 28 confirmed cases of botulism — a rare but potentially life-threatening condition.
One of the victims, a 36-year-old mother of three, was left in intensive care after receiving what she believed were routine anti-wrinkle injections. She had paid £75 for three injections, half the price of her previous visit. Within days, she began experiencing vision problems and was diagnosed with ptosis — a condition that causes drooping of the eyelid. Her health deteriorated rapidly, requiring emergency intervention and anti-toxin therapy. These cases have triggered a public health investigation led by the UK Health Security Agency and raised urgent questions about the legal safeguards surrounding aesthetic procedures.
Prescription-only regulation update
The practitioner’s conduct in this case constitutes multiple breaches of UK law. Botulinum toxin is classified as a prescription-only medicine (POM), which may only be prescribed and administered by qualified healthcare professionals. The use of an unlicensed product by an unqualified individual not only violates medicines legislation but also exposes patients to serious harm.
This Nursing and Midwifery Council (NMC) introduced a new regulation on 1st June 2025, requiring all nurse and midwife prescribers to carry out face-to-face consultations before prescribing POMs for non-surgical cosmetic procedures. The regulation was designed to eliminate remote prescribing practices that had allowed unqualified individuals to access and misuse prescription drugs. It followed research showing that many patients were unaware these treatments involved prescribed medicines, and that procedures were often carried out in unregulated environments by non-qualified individuals. While some stakeholders had advocated for video consultations, the NMC prioritised clinical safety over convenience.
Continued breaches
Despite this regulatory step forward, reports suggest that some practitioners are already finding ways to circumvent the rules. These include using proxy prescribers who never meet the patient, conducting superficial or sham consultations, and working in poorly monitored or entirely unregulated settings. Such practices undermine the intent of the regulation and continue to place patients at risk.
It is important for all prescribers to be aware of the dangers, not only to patient safety but also to their own professional accountability. Prescribing without proper clinical assessment, or enabling others to misuse prescriptions, can result in regulatory sanctions, civil liability and even criminal prosecution.
Regulation without oversight is ineffective. Even with the new rules in place the aesthetic practitioner in this case was able to obtain and administer a dangerous, unlicensed product, demonstrating the urgent need for coordinated action across regulatory bodies, law enforcement and public health agencies.
Victims of malpractice in these circumstances may pursue civil claims for negligence, seeking compensation for both physical and psychological harm. The Consumer Protection Act 1987 may also offer legal recourse against suppliers or distributors of illegal products, if they can be identified. The rarity of botulinum toxicity only highlights the severity of the situation and the importance of preventative legal safeguards.
More reform required?
Beyond the legal implications, this case raises ethical concerns about the commodification of medical treatments within the beauty industry. The pursuit of low-cost cosmetic enhancements often leads consumers to unregulated providers who operate outside the scope of professional accountability. Without mandatory licensing and clear standards of practice, the public remains vulnerable to harm from individuals lacking the necessary training, qualifications and ethical obligations.
Comprehensive reform is urgently needed. This must include mandatory registration and licensing of all aesthetic practitioners, tighter controls on the import and distribution of botulinum toxin products, strong anticounterfeiting controls, and robust public education campaigns to raise awareness of the risks associated with unregulated treatments. Real-time reporting systems for adverse events could also play a vital role in early detection and prevention, allowing regulators to intervene before harm escalates.
Until these measures are fully implemented and enforced, cases like this will continue to pose a serious threat to public health. The law must not only respond to such incidents but evolve to prevent them, ensuring that aesthetic medicine is practiced safely, ethically and within the bounds of professional regulation.
Talk to us
Ensuring compliance with healthcare regulation and professional standards has never been more important — particularly where patient safety and public confidence are at risk. Our Regulatory and Professional Conduct team supports practitioners, prescribers and healthcare providers in navigating complex regulatory frameworks, managing professional accountability and responding to enforcement or fitness to practise concerns.
If you’d like more information about your regulatory obligations or how we can support your practice, please get in touch by emailing thorrun.govind@brabners.com or hannah.fawcett@brabners.com.
Talk to us by giving us a call on 0333 004 4488, sending us an email at hello@brabners.com or completing our online form.

Hannah Fawcett
Hannah is a Partner and Chartered Trade Mark Attorney. She leads our beauty and fashion team.
Read more
Talk to us
Loading form...